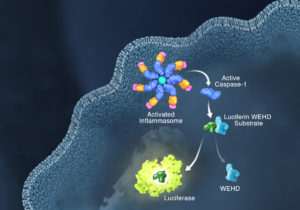
Innate immunity, the first line of immune defense, uses a system of host pattern recognition receptors (PRRs) to recognize signals of “danger” including invariant pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). These signals in turn recruit and assemble protein complexes called inflammasomes, resulting in the activation of caspase-1, the processing and release of the pro-inflammatory cytokines IL-1ß and IL-18, and the induction of programmed, lytic cell death known as pyroptosis.
Innate immunity and the activity of the inflammasome are critical for successful immunity against a myriad of environmental pathogens. However dysregulation of inflammasome activity is associated with many inflammatory diseases including type 2 diabetes, obesity-induced asthma, and insulin resistance. Recently, aberrant NLRP3 inflammasome activity also has been associated with age-related macular degeneration and Alzheimer disease. Understanding the players and regulators involved in inflammasome activity and regulation may provide additional therapeutic targets for these diseases.
Currently inflammasome activation is monitored using antibody-based techniques such as Western blotting or ELISA’s to detect processed caspase-1 or processed IL-1ß. These techniques are tedious and are only indirect measures of caspase activity. Further, gaining information about kinetics—relating inflammasome assembly, caspase-1 activation and pyroptosis in time—is very difficult using these methods. O’Brien et al. describe a one-step, high-throughput method that enables the direct measurement of caspase-1 activity. The assay can be multiplexed with a fluorescent viability assay, providing information about the timing of cell death and caspase-1 activity from the same sample.
 The bioluminescent caspase-1 assay, was used to assess caspase-1 activation in response to known inflammasome activators in several model systems. The authors were able to demonstrate, using a specific caspase-1 inhibitor, that the assay is specific for caspase-1 activity. To show that the caspase-1 activity measured by the assay was biologically relevant, they measured caspase-1 activity, IL-1ß release and cell death concurrently in PMA-differentiated and undifferentiated THP-1 cells. PMA stimulates THP-1 monocytes to differentiate to a more macrophage-like phenotype. They were able to show that cell death occurred in differentiated cells treated with all of the cytotoxic agents but that only the known inflammasome activators (α-hemolysin, LPS, and nigericin) induced caspase-1 activity. Further IL-1ß was detected in the cell medium of the differentiated cells when cell death occurred, but not in medium from undifferentiated cells, which have not had proIL-1b upregulated. In undifferentiated monocytes, the inflammasome inducers, nigericin and a-hemolysin, triggered caspase-1 activity and pyroptosis in the absence of any IL-1b.
The bioluminescent caspase-1 assay, was used to assess caspase-1 activation in response to known inflammasome activators in several model systems. The authors were able to demonstrate, using a specific caspase-1 inhibitor, that the assay is specific for caspase-1 activity. To show that the caspase-1 activity measured by the assay was biologically relevant, they measured caspase-1 activity, IL-1ß release and cell death concurrently in PMA-differentiated and undifferentiated THP-1 cells. PMA stimulates THP-1 monocytes to differentiate to a more macrophage-like phenotype. They were able to show that cell death occurred in differentiated cells treated with all of the cytotoxic agents but that only the known inflammasome activators (α-hemolysin, LPS, and nigericin) induced caspase-1 activity. Further IL-1ß was detected in the cell medium of the differentiated cells when cell death occurred, but not in medium from undifferentiated cells, which have not had proIL-1b upregulated. In undifferentiated monocytes, the inflammasome inducers, nigericin and a-hemolysin, triggered caspase-1 activity and pyroptosis in the absence of any IL-1b.
When the researchers looked at the timing between activation of caspase-1 and the onset of cell death, they found that pyroptosis was detected within 30–60 minutes of caspase-1 activity in the cell lines investigated, consistent with caspase-1 triggering cell death. To determine whether blocking caspase-1 would prevent pyroptosis, the authors used a pan caspase inhibitor (Z-VAD-FMK) and a specific caspase-1 inhibitor (Z-YVAD-FMK) and pretreated differentiated and undifferentiated THP-1 cells before inducing the inflammasome with LPS, -hemolysin or nigericin. At the time of peak caspase-1 activity, Z-VAD-FMK inhibited all IL-1ß release and significantly blocked cell death in the inflammasome-activated differentiated cells, and it inhibited cell death in nigericin- and α–hemolysin–treated undifferentiated cells. Surprisingly, Z-YVAD-FMK had little effect on cell death and IL-1ß release, even though the researchers could show that it was functional since it inhibited all caspase-1 activity in the cell medium. These unexpected results led to additional kinetic studies using additional inhibitors.
| Cell Death | Il-1ß release | ||||
| pan inhibitor | specific inhibitor | pan inhibitor | specific inhibitor | ||
| Undifferentiated monocytes | Unstimulated (neg. control) | na | na | na | na |
| LPS | na | na | na | na | |
| α-hemolysin | inhibited | slight inhibition | na | na | |
| nigericin | inhibited | slight inhibition | na | na | |
| ionomycin (+ death control) | no effect | no effect | na | na | |
| Differentiated monocytes (macrophage-like) | Unstimulated (neg. control) | na | na | na | na |
| LPS | slight inhibition | no effect | inhibited | slight inhibition | |
| α-hemolysin | inhibited | no effect | inhibited | no effect | |
| nigericin | inhibited | no effect | inhibited | slight inhibition | |
| Ionomycin (+ death control) | no effect | no effect | no effect | no effect | |
To learn more about the role of caspase-1 in pyroptosis, the authors of the paper used the IL-1ß-independent model of undifferentiated THP-1 cells treated with nigericin to induce the inflammasome. They pretreated the cells with different doses of either the pan caspase inhibitor Z-VAD-FMK, one of two specific caspase-1 inhibitors (Z-YVAD-FMK or VX-756) or a cathepsin B inhibitor (thought to function upstream of caspase-1). They monitored for cell death up to six hours or up to 21 hours. Data indicated that all the inhibitors, in a dose-dependent manner, delayed cell death in this model, but none of them protected the cells completely from cell death. Inhibiting caspase-1 activity only alters the timing of cell death in inflammasome-activated THP-1 cells.
Using a one-step, direct enzyme activity assay for caspase-1 activity enables a better understanding of the kinetics of inflammasome activation from signal through caspase activity to pyroptosis. This will enable more detailed dissection of the regulators of this process and perhaps lead to a better understanding of the players in this signaling pathway that might serve as therapeutic targets for inflammatory-related diseases.
Learn more about the Caspase-Glo® 1 Inflammasome Assay, now available in bulk size (100ml), ideal for use in high-throughput screening.
Further Reading
O’Brien, M. et al. (2017) A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells J. Immunol. Meth. 447, 1–13.
Shroder, K. and Tschopp, J. (2010) The Inflammasomes Cell 140, 821–832.
Lamkanfi, M and Dixit, V.M. (2014) Mechanisms and Functions of Inflammasomes Cell 157, 1013–1022.
Michele Arduengo
Latest posts by Michele Arduengo (see all)
- An Unexpected Role for RNA Methylation in Mitosis Leads to New Understanding of Neurodevelopmental Disorders - March 27, 2025
- Unlocking the Secrets of ADP-Ribosylation with Arg-C Ultra Protease, a Key Enzyme for Studying Ester-Linked Protein Modifications - November 13, 2024
- Exploring the Respiratory Virus Landscape: Pre-Pandemic Data and Pandemic Preparedness - October 29, 2024